Danyelza: Uses, Side Effects, How it is Administered, Price
Danyelza is a prescription medicine used in combination with a medicine called granulocyte-macrophage colony-stimulating factor (GM-CSF) to treat children 1-year of age and older and adults with high-risk neuroblastoma in the bone or bone marrow that: has come back (relapsed) or that did not respond to previous treatment (refractory), and has shown a partial response, minor response, or stable disease to prior therapy. Neuroblastoma is a type of pediatric cancer that develops in the nervous system of babies and young children. Neuroblastoma grows in immature nerve tissue (neuroblasts).
Danyelza was approved by the FDA on the 25th of November, 2020 based on two clinical studies that looked at reducing tumor size. Danyelza is still being studied to confirm the study results and the clinical benefit of treatment.
How is Danyelza Administered?
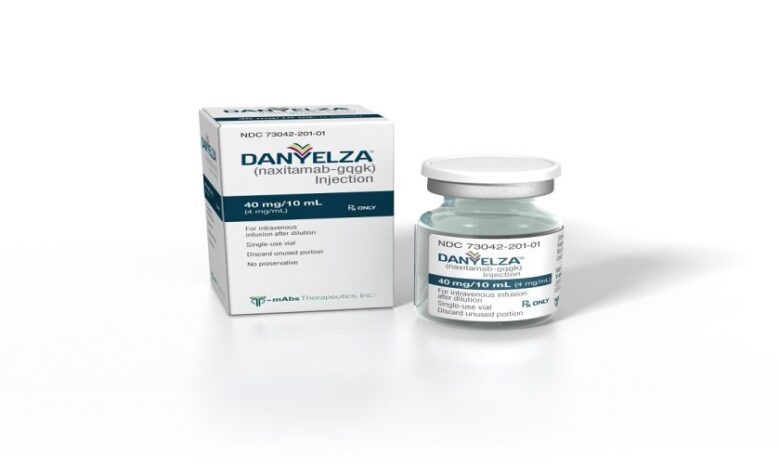
Danyelza Injection comes as : 40 mg/10 mL (4 mg/mL) in a single-dose vial. The recommended dosage of Danyelza is 3 mg/kg/day (up to 150 mg/day), administered as an intravenous infusion after dilution on Days 1, 3, and 5 of each treatment cycle. Treatment cycles are repeated every 4 weeks until complete response or partial response, followed by 5 additional cycles every 4 weeks. Subsequent cycles may be repeated every 8 weeks.
What is the most important information I should know about Danyelza?
Danyelza may cause serious side effects, including:
• Serious infusion-related reactions. Danyelza can cause serious infusion-related reactions that require immediate medical attention. Infusion-related reactions are common with Danyelza. Tell your healthcare provider right away if you get any signs or symptoms during or after your Danyelza infusion, including:
o swelling of your face, eyes, lips, mouth, or tongue
o itching
o redness on your face (flushing)
o skin rash or hives
o trouble breathing
o cough or wheezing
o noisy high-pitched breathing
o feeling faint or dizziness (low blood pressure)
• Nervous system problems. Talk to your healthcare provider right away if you have new symptoms or worsening of nervous system problems, including:
o Severe pain from nerves (neuropathic pain), including pain in the belly (abdomen), bone, neck, legs, or arms. Pain is common with Danyelza and can be severe.
o Inflammation of the spinal cord. Signs or symptoms may include:
- Weakness in your legs or arms
- Bladder and bowel problems
- Pain in back, legs, or stomach (abdomen)
- Numbness
- Tingling
- Burning sensation
o Reversible Posterior Leukoencephalopathy Syndrome (RPLS – also known as Posterior Reversible Encephalopathy Syndrome – PRES). PRES is a condition that affects the brain. Your healthcare provider will monitor your blood pressure and check for any neurologic symptoms after your Danyelza infusion. Signs or symptoms of PRES may include:
- Severe headache
- Vision changes
- Changes in mental status, such as confusion, disorientation, or decreased alertness
- Difficulty speaking
- Weakness in your arms or legs
- Seizures
Numbness, tingling, or burning sensation in the arms or legs.
o Nervous system problems of the eye. Signs or symptoms may include:
- Unequal pupil size
- Blurred vision
- Trouble focusing your eyes
- Larger pupil size (dilated)
- Decreased ability to see
- Sensitivity to light
o Problems urinating or emptying your bladder (prolonged urinary retention).
Do not receive Danyelza if you have had a severe allergic reaction to naxitamab-gqgk (the active ingredient in Danyelza). Ask your healthcare provider if you are not sure.
Before receiving Danyelza, tell your healthcare provider about all your medical conditions, including if you:
• have high blood pressure
• are pregnant or plan to become pregnant. Danyelza may harm your unborn baby.
o Your healthcare provider will do a pregnancy test before you start treatment with Danyelza.
o Females who are able to become pregnant should use effective birth control (contraception) during treatment and for 2 months after your last dose of Danyelza. Talk to your healthcare provider about birth control choices that may be right for you during this time.
o Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with Danyelza.
• are breastfeeding or plan to breastfeed. It is not known if Danyelza passes into your breast milk. Do not breastfeed during treatment and for 2 months after your last dose of Danyelza.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of Danyelza?
Danyelza may cause serious side effects, including:
• High blood pressure (hypertension). High blood pressure is common in people who receive Danyelza. Your blood pressure will be monitored during your Danyelza infusion, and at least each day on Days 1 to 8 of each Danyelza treatment cycle. Tell your healthcare provider right away if you get any signs or symptoms of high blood pressure, including:
o headaches
o seizures
o nausea or vomiting
o chest pain
o dizziness
o visual changes
o shortness of breath
o feeling that your heart is pounding or racing (palpitations)
o nose bleeds
The most common side effects of Danyelza include:
• fast heart rate
• vomiting
• cough
• nausea
• decreased white blood cell, red blood cell, and platelet counts
• diarrhea
• decreased appetite
• tiredness
• skin rashes
• decreased level of potassium, sodium, and phosphate in the blood
• hives
• fever
• headache
• injection site reaction
• swelling of the body or only in one part of the body
• anxiety
• irritability
• increased liver function blood tests
• decreased blood sugar level
• decreased calcium levels in the blood
• decreased protein levels (albumin) in the blood
These are not all of the possible side effects of Danyelza. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How much does Danyelza cost?
4 mg/mLDanyelza intravenous solution
from $21,273.69 for 10 milliliters
| Quantity | Per unit | Price |
| 10 milliliters | $2,127.37 | $21,273.69 |