News & Alerts
-
Jan- 2024 -12 January

ResMed Ltd. Recalls CPAP Masks with Magnets Over Magnetic Interference Concerns: FDA Issues Class I Recall
In a recent development, ResMed Ltd. has initiated a recall of its Continuous Positive Airway Pressure (CPAP) masks with magnets,…
Read More » -
11 January

BioArctic’s Partner Eisai to Discuss Marketing Authorization for Lecanemab with EMA’s Scientific Advisory Group
BioArctic AB’s (Nasdaq Stockholm: BIOA B) partner Eisai has announced that the Scientific Advisory Group (SAG) will convene to discuss…
Read More » -
11 January
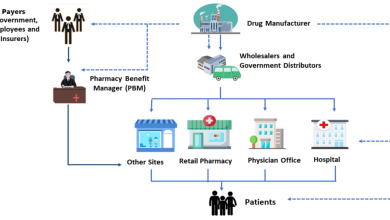
Growth in the Pharmaceutical Traceability Market to Reach $17.92 Billion from 2023-2028
The global pharmaceutical traceability market is anticipated to witness substantial growth, projecting an increase of USD 17.92 billion from 2023…
Read More » -
11 January

SyntheticGestalt and Enamine Forge Collaboration to Revolutionize Drug Discovery with AI
SyntheticGestalt, a cutting-edge research and development company specializing in applying artificial intelligence (AI) to the life sciences, has announced a…
Read More » -
11 January

AbbVie Confident in Botox Market Share Amid Rising Competition
In the face of intensifying competition in the aesthetics space, pharmaceutical giant AbbVie (ABBV.N) has expressed confidence in maintaining Botox’s…
Read More » -
11 January

US FDA Temporarily Allows Import of French Syphilis Drug to Address Shortages
In response to the alarming shortage of syphilis treatment drugs in the United States, the U.S. Food and Drug Administration…
Read More » -
9 January

FDA Issues Warning on Toxic Tejocote Root Supplements Contaminated with Yellow Oleander
In September 2023, the Centers for Disease Control and Prevention published a report of several tejocote root products found to…
Read More » -
9 January

Megadyne Medical Products, Inc. Issues Recall for Mega Soft Universal Patient Return Electrode Due to Burn Risks
Megadyne Medical Products, Inc. has initiated a recall of its Mega Soft Universal Patient Return Electrode, Mega Soft Universal Dual,…
Read More » -
9 January

FDA Approves Vaporized Hydrogen Peroxide as Established Sterilization Method for Medical Devices
In a significant development, the U.S. Food and Drug Administration (FDA) has officially recognized vaporized hydrogen peroxide (VHP) as an…
Read More » -
9 January

Chiesi Group and Oak Hill Bio Collaborate on OHB-607, a Neonatal Therapy
Chiesi Group, an international biopharmaceutical and healthcare group, and Oak Hill Bio, a neonatology and rare disease therapeutics company, have…
Read More »
