Beyfortus: Uses, Benefits, Dosage, Side Effects, Interactions
Beyfortus, also known as nirsevimab-alip, is a long-acting monoclonal antibody designed to combat the respiratory syncytial virus (RSV). This medication serves as protection for neonates and infants against lower respiratory tract disease (LRTD) caused by RSV.
It is specifically intended for use in neonates and infants born during or entering their first RSV season to shield them from RSV-induced lower respiratory tract infections. Additionally, it provides protection for vulnerable infants during their second RSV season, such as those with congenital heart disease or chronic lung disease.
The mechanism of action for Beyfortus involves attaching to a protein on the surface of the RSV called the F protein. By doing so, it prevents the virus from entering cells, especially those in the lungs. This attachment inhibits the essential membrane fusion step in the viral entry process, neutralizing the virus, and blocking cell-to-cell fusion. The drug is produced using recombinant DNA technology and is classified as a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody. It possesses a triple amino acid substitution in the Fc region, which enhances its half-life and prolongs its effectiveness.
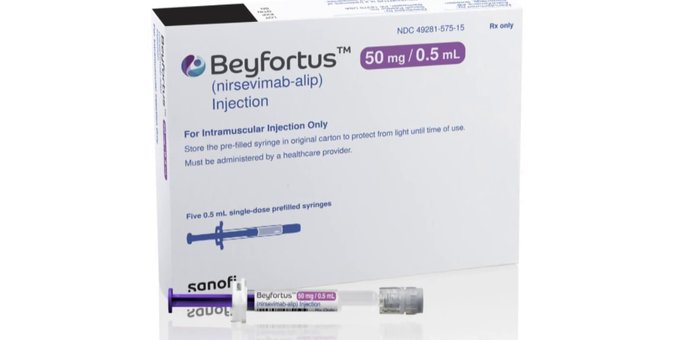
Beyfortus is available in two different strengths, each contained in a pre-filled syringe with a distinct plunger rod color for easy identification:
1. 50mg strength: It comes in a 0.5 mL pre-filled syringe with a purple plunger rod.
2. 100mg strength: This version is supplied in a 1 mL pre-filled syringe with a light blue plunger rod.
Apart from the active ingredient, nirsevimab-alip, Beyfortus also contains the following inactive ingredients to help stabilize and deliver the medication:
• L-histidine
• L-histidine hydrochloride
• L-arginine hydrochloride
• Sucrose
• Polysorbate 80
• Water for injections
These inert substances do not contribute to the therapeutic effect of Beyfortus but play essential roles in maintaining its stability and ensuring safe administration. Always consult the package insert or your healthcare provider for a complete list of ingredients and possible allergies before using Beyfortus.
Beyfortus received FDA approval on July 17, 2023. However, it is important to be cautious when administering the drug, as serious hypersensitivity reactions, including anaphylaxis, have been reported with other human IgG1 monoclonal antibodies.
Benefits
Beyfortus (nirsevimab-alip) offers several significant benefits in protecting neonates and infants against lower respiratory tract disease (LRTD) caused by the respiratory syncytial virus (RSV). Some of the key benefits of Beyfortus include:
1. Long-lasting protection: Beyfortus is a long-acting monoclonal antibody with an extended half-life, meaning it stays effective for a longer duration compared to some other treatments. This allows for sustained protection against RSV for several months after administration.
2. Targeted mechanism of action: The drug works by attaching to a specific protein on the surface of the RSV called the F protein. This targeted action inhibits the virus from entering cells, particularly in the lungs, preventing RSV infection and its associated complications.
3. Prevention of severe RSV disease: Beyfortus helps prevent lower respiratory tract infections caused by RSV, which can be particularly severe and life-threatening in neonates and infants. By neutralizing the virus and blocking its replication, Beyfortus reduces the risk of severe RSV-related illness.
4. Protection for vulnerable populations: Beyfortus is especially beneficial for vulnerable infants, including those born during or entering their first RSV season and those with conditions like congenital heart disease or chronic lung disease. These populations are at a higher risk of severe RSV disease, and Beyfortus provides an extra layer of protection for them.
5. Convenience of administration: The drug is administered through intramuscular injection, which can be performed by healthcare professionals. This mode of administration makes it relatively straightforward to deliver the treatment to infants in need.
6. Compatibility with vaccines: Beyfortus can be safely administered alongside childhood vaccines, making it easier to provide multiple preventive measures to infants simultaneously.
7. Developed in partnership: The collaboration between Astra Zeneca and Sanofi in creating Beyfortus demonstrates the commitment of both pharmaceutical companies to address the challenges posed by RSV infections and improve infant health.
Dosage
Beyfortus is administered through an intramuscular injection, typically into the anterolateral aspect of the thigh, which is the top, outer part of the thigh. Healthcare professionals are responsible for performing this injection to ensure proper delivery and safety. The gluteal muscle is not used as a routine injection site due to the risk of damaging the sciatic nerve.
For infants born outside the respiratory syncytial virus (RSV) season, a single administration of Beyfortus is given before the commencement of the RSV season. It’s important to note that the protection offered by Beyfortus lasts for approximately 5 months.
Beyfortus can be administered concurrently with childhood vaccines, but it is crucial not to mix the drug with any vaccines or other medications in the same syringe or vial.
The dosing of Beyfortus depends on specific criteria:
1. Neonates and infants born during or entering their first RSV season:
• For those weighing less than 11lb (<5kg), a 50mg dose is given via an intramuscular injection using a 0.5mL prefilled syringe.
• For those weighing 11lb (≥5kg) or more, a 100mg dose is given via an intramuscular injection using a 1mL prefilled syringe.
2. Vulnerable children at increased risk for severe RSV disease in their second RSV season:
• A single 200mg dose is administered as two intramuscular injections (2 x 100 mg).
3. Infants undergoing cardiac surgery with cardiopulmonary bypass:
• An additional dose may be given once the infant is stabilized after surgery, depending on the length of time passed and the number of RSV seasons the infant has been exposed to.
• For the first RSV season, if it has been less than 90 days since the first dose, either 50mg or 100mg is administered based on body weight.
• If it has been 90 days or more since the first dose, a single dose of 50mg is given regardless of body weight.
• For the second RSV season, if it has been less than 90 days since the first dose, 200mg is administered regardless of body weight.
• If it has been 90 days or more since the first dose, 100mg is given regardless of body weight.
Please note that there is limited data available for the use of Beyfortus in extremely preterm infants (gestational age <29 weeks) who are less than 8 weeks old. Always follow the dosing instructions provided by a qualified healthcare professional to ensure proper administration and optimal efficacy of Beyfortus.
Side effects of Beyfortus
Side effects of Beyfortus may include:
1. Allergic reaction: Seek emergency medical help if your child experiences symptoms like hives, difficulty breathing, or swelling of the face, lips, tongue, or throat.
2. Mild-to-moderate rash: Approximately 0.7% of infants may develop a mild-to-moderate rash within 14 days after receiving Beyfortus.
3. Fever: Around 0.3% to 0.5% of infants may experience fever within 7 days post-administration.
4. Non-serious injection site reactions: Some infants, about 0.3% to 0.5%, may experience non-serious reactions at the injection site within 7 days after the dose.
Please note that this is not an exhaustive list, and other side effects may occur. If you notice any unusual or concerning symptoms, it is essential to seek advice from a healthcare professional promptly.
Interactions
Drug that can interact with Beyfortus include:
1. Beyfortus does not interfere with reverse transcriptase polymerase chain reaction (RT-PCR) or rapid antigen detection RSV diagnostic assays that use commercially available antibodies targeting antigenic site I, II, or IV on the RSV fusion (F) protein.
2. It is recommended to confirm immunological assay results with an RT-PCR-based assay when clinical observations are consistent with RSV infection but the immunological assay shows negative results.
3. This list is not exhaustive. Beyfortus may interact with other drugs, including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible drug interactions are listed here.
Please consult a healthcare professional or pharmacist for comprehensive information on potential drug interactions with Beyfortus and any medications or supplements your child may be taking.
Storage instructions for Beyfortus
- Store refrigerated between 36°F to 46°F (2°C to 8°C).
- Beyfortus may be kept at room temperature (68°F to 77°F or 20°C to 25°C) for a maximum of 8 hours.
- After removing Beyfortus from the refrigerator, use it within 8 hours or discard it.
- Store the medication in its original carton to protect it from light until the time of use.
- Do not freeze Beyfortus.
- Do not shake the medication.
- Avoid exposing Beyfortus to excessive heat.
Following these storage guidelines is essential to ensure the medication’s stability and effectiveness. Always check the expiration date and discard any expired or damaged Beyfortus according to proper disposal guidelines. If you have any concerns about storage or handling, consult your healthcare provider or pharmacist for clarification.