Drug News
Pfizer COVID Vaccine For Children 5 Things You Should Know
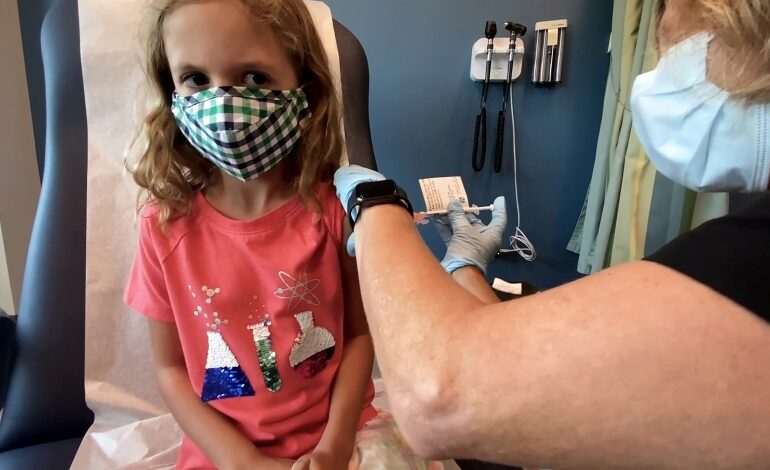
The Centers for Disease Control and Prevention, the national public health agency of the United States through its Director Rochelle Walensky, on Nov. 2 endorsed the agency advisory panel’s recommendation that children ages 5-11 be vaccinated against COVID-19 with Pfizer’s vaccine, meaning providers can begin inoculating kids in the pediatric age group immediately. Here are five things parents should know about pediatric COVID-19 vaccinations:
- Clinical trials have shown the vaccine to be 90.7 percent effective in children ages 5-11 and that the immune response was comparable to people ages 16 to 25. The vaccine’s safety was studied in approximately 3,100 children ages 5-11, and no serious side effects have been detected in the ongoing clinical trial.
- Pfizer’s COVID-19 vaccine for children ages 5-11 is 10 micrograms, which differs from the 30-microgram dose for everyone older than 12. The vaccine is administered in two doses, three weeks apart.
- Children can receive COVID-19 vaccines at pediatricians’ offices, pharmacies, children’s hospitals, rural health clinics and school- and community-based clinics.
- Vaccine providers are required to administer shots for free, regardless of a patient’s insurance status.
- The U.S. has purchased enough of Pfizer’s COVID-19 vaccine to inoculate the country’s 28 million children ages 5-11 with the appropriate dosage.