What is an NDC code?
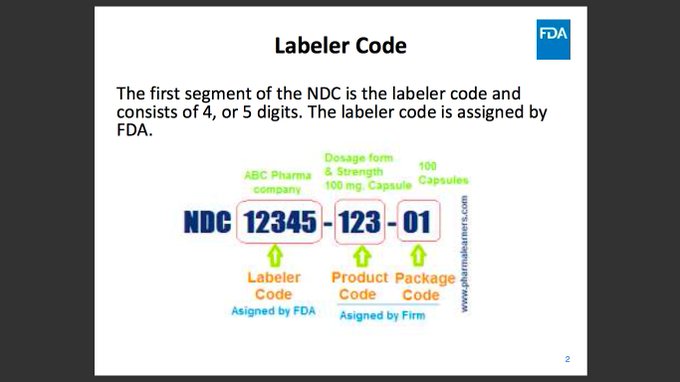
The national drug code (NDC) is a unique product identifier used in the United States for drugs intended for human use. Each listed drug product is assigned a unique 10-digit, 3-segment number. This number, known as the NDC, identifies the labeler, product, and trade package size. The first segment, the labeler code, is assigned by the FDA. The second set of numbers is the product code, which identifies the specific strength, dosage form (i.e, capsule, tablet, liquid), and formulation of a drug for a specific labeler. Finally, the third set is the package code, which identifies package sizes and types. The NDC code can be found on the outside packaging of the drug.
Section 510 of the Federal Food, Drug and Cosmetic Act (Act), 21 U.S.C. §360, requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or processed by it for commercial distribution. Changes in the Act, resulting from the Food and Drug Administration Amendments Act of 2007 (Public Law 110-85) (FDAAA) require that drug establishment registration and drug listing information be submitted electronically unless a waiver is granted.
Drug products are identified and reported using a unique, three-segment number, called the National Drug Code (NDC), which is a universal product identifier for human drugs. FDA inputs the full NDC number and the information submitted as part of the listing process into a database known as the Drug Registration and Listing System (DRLS), which is transforming into the electronic system (eDRLS). The information submitted as part of the listing process, the NDC number, DRLS, eDRLS, and the NDC Directory, are used in the implementation and enforcement of the Act.
What products are included in the NDC Directory?
The current edition of the NDC Directory is limited to prescription drugs, OTC drugs, and insulin products that have been manufactured, prepared, propagated, compounded, or processed by registered establishments for commercial distribution. The products have been listed in accordance with the Drug Listing Act and regulatory provisions concerning the submission of drug product information to FDA.
Why are some drug products not in the NDC Directory?
There are a number of reasons why a drug product may not appear in the NDC Directory, such as:
- the product may not be a prescription drug, OTC, or an insulin product
- the firm has notified the FDA that the product is no longer being marketed;
- the firm has not complied fully with its listing obligations and therefore its product is not included until complete information is provided.
The inclusion of a firm or its products in the NDC Directory does not denote approval by the FDA of the firm or any of its marketed products, nor is it a determination that a product is a drug as defined by the act, nor does it denote that a product is covered by or eligible for reimbursement by Medicare, Medicaid, or other payers.
How to find an NDC number for a drug?
You can easily use ONLINE pill identification tools to identify medications by NDC number. The FDA also maintains a searchable database of NDC codes on its website. NDC numbers can also be found in the drug product labeling (for example, the package insert) as well as on the package itself.





