FDA Grants Over-the-Counter Status to Opill (norgestrel): A Daily Oral Contraceptive
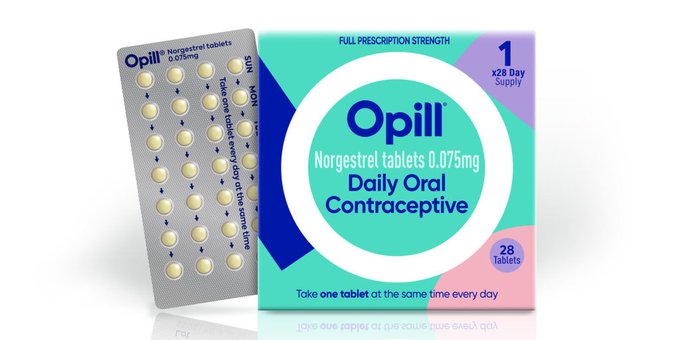
The U.S. Food and Drug Administration (FDA) has granted approval for the first over-the-counter birth control pill in the country. This significant development is expected to greatly enhance contraception accessibility for Americans. The FDA announced that women will be able to purchase the progestin-only oral contraceptive at various retail locations, including drug stores, convenience stores, and grocery stores. Notably, there are no age restrictions for purchasing the pill.
Manufactured by Perrigo, the birth control pill, known as Opill, is anticipated to hit store shelves in January or February, with the suggested retail price to be disclosed in the upcoming autumn. Opill initially received FDA approval in 1973, while other types of birth control pills will remain available by prescription only.
Dr. Patrizia Cavazzoni, the director of the FDA’s Center for Drug Evaluation and Research, expressed enthusiasm about the approval, stating that it is the first time a nonprescription daily oral contraceptive will be accessible to millions of people in the United States. The FDA believes that when used correctly, daily oral contraception is safe and more effective in preventing unintended pregnancy compared to existing nonprescription contraceptive methods.
Leading medical organizations, such as the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists (ACOG), as well as other medical associations, have already voiced their support for over-the-counter access to hormonal contraception without age limitations.
The Free the Pill coalition, which consists of numerous advocacy groups working towards over-the-counter status for birth control pills, has been advocating for this change since 2004. The coalition highlights the barriers faced by individuals, particularly those from marginalized communities, who seek to use birth control pills.
This landmark decision by the FDA aligns with the unanimous recommendation of an advisory panel, which endorsed the over-the-counter pill in May. In its briefing documents before the panel meeting, the FDA expressed concerns regarding the proper use of these pills.
According to the FDA, studies have indicated that a significant portion of consumers demonstrate a good understanding of the label instructions for Opill, supporting their ability to use the drug appropriately when purchased over the counter. The FDA initially considered Opill for over-the-counter status in November 2022 but delayed the decision to review additional information.
Dr. Kristyn Brandi, an obstetrician/gynecologist in Newark, N.J., and ACOG’s Darney-Landy Fellow, believes that Opill should be available for purchase without a prescription. The primary contraindication for this pill is having active breast cancer. However, Dr. Brandi pointed out that individuals with active breast cancer typically consult multiple healthcare providers who would discuss the use of birth control with them.
The FDA expressed concerns that the pill may be less effective in people who are overweight or obese. However, Dr. Brandi does not consider this to be a significant issue. She stated that there are no different precautions or guidelines for patients who are obese and take the pill through a prescription.
The availability of a birth control pill without a prescription is particularly important following the U.S. Supreme Court’s decision last year to overturn Roe v. Wade, thereby eliminating the constitutional right to terminate pregnancies and leaving the matter to individual states. Dr. Brandi emphasized that individuals already face barriers to reproductive healthcare, and while over-the-counter access to contraception does not solve the issue of abortion bans, it does increase access to contraception, enabling more people to prevent pregnancies. The value of this cannot be overstated.